Chapter 4 - Materials: Metals and Non-metals Exercise 53
Solution 1
(a) Zinc
Concept insight: Metals are malleable.
Concept insight: Metals are malleable.
Solution 2
(c) Generally, metals are ductile
However, mercury metal - a liquid at room temperature - cannot be drawn into wires and is not ductile.
Concept insight: Ductility means that substance can be drawn into wires.
However, mercury metal - a liquid at room temperature - cannot be drawn into wires and is not ductile.
Concept insight: Ductility means that substance can be drawn into wires.
Chapter 4 - Materials: Metals and Non-metals Exercise 54
Solution 1
(a) reactive.
(b) Good, electricity
(c) more
(d) hydrogen.
(b) Good, electricity
(c) more
(d) hydrogen.
Solution 2
(i) Generally, non-metals react with acids. (F)
Concept insight: non metallic oxides are acidic in nature.
(ii) Sodium is a very reactive metal. (T)
(iii) Copper displaces zinc from zinc sulphate solution. (F)
Concept insight: in a displacement reaction more reactive element displaces less reactive metal from its salt.
(iv) Coal can be drawn into wires. (F)
Concept insight: Coal is a form of carbon which is a non metal.
Concept insight: non metallic oxides are acidic in nature.
(ii) Sodium is a very reactive metal. (T)
(iii) Copper displaces zinc from zinc sulphate solution. (F)
Concept insight: in a displacement reaction more reactive element displaces less reactive metal from its salt.
(iv) Coal can be drawn into wires. (F)
Concept insight: Coal is a form of carbon which is a non metal.
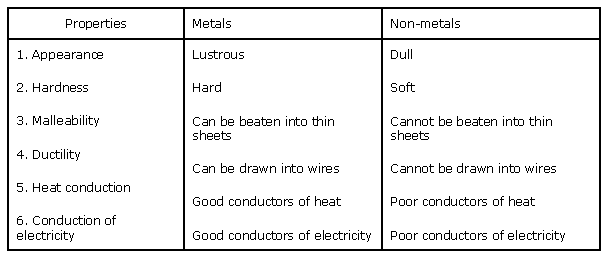
Solution 3
Solution 4
(a) Aluminium foils are used to wrap food items because aluminium metal is malleable. Therefore, it can be beaten into thin foils.
Concept insight: metals are malleable.
(b) Metals are good conductors of heat and electricity. Therefore, immersion rods for heating liquids are made of metallic substances.
(c) A metal can displace a less reactive metal from its salt in an aqueous solution. But zinc is more reactive than copper. Therefore, copper cannot displace zinc from its salt solution.
Cu(s) + ZnSO4 (aq) No reaction
No reaction
Concept insight: in a displacement reaction more reactive element displaces less reactive elment.
(d) Sodium and potassium are stored in kerosene because they are highly reactive elements. They can easily catch fire even when in contact with air.
Concept insight: sodium and potassium are so highly reactive that they react with water vapour present in air. So they are stored in kerosene.
Concept insight: metals are malleable.
(b) Metals are good conductors of heat and electricity. Therefore, immersion rods for heating liquids are made of metallic substances.
(c) A metal can displace a less reactive metal from its salt in an aqueous solution. But zinc is more reactive than copper. Therefore, copper cannot displace zinc from its salt solution.
Cu(s) + ZnSO4 (aq)
Concept insight: in a displacement reaction more reactive element displaces less reactive elment.
(d) Sodium and potassium are stored in kerosene because they are highly reactive elements. They can easily catch fire even when in contact with air.
Concept insight: sodium and potassium are so highly reactive that they react with water vapour present in air. So they are stored in kerosene.
Solution 5
As lemon pickle contains acid so it can not be stored in metallic vessels as acids readily react with metals.
Concept insight: acids react with metals and produce salt and hydrogen.
Concept insight: acids react with metals and produce salt and hydrogen.
Solution 6

Chapter 4 - Materials: Metals and Non-metals Exercise 55
Solution 1

Solution 2

Solution 3
To polish a gold ornament, it is dipped in a liquid called aquaregia (a mixture of hydrochloric acid and nitric acid). On getting the environment of aquaregia, the outer layer of gold dissolves and the inner shiny layer appears. The dissolving of the layer causes a reduction in the weight of the jewellery.
Comments
Post a Comment